In recent years, the healthcare industry has advanced exponentially in terms of science, technology, and the sophistication of solutions. Precision therapies, connected devices, artificial intelligence, and new models of care are already a reality. Still, as innovation accelerates, market access has emerged as one of the sector’s main strategic bottlenecks. And the challenge is no longer purely technological.
In many cases, solutions are mature, validated, and ready for use; the critical issue lies in how these innovations move along the path from development to incorporation, financing, and large-scale adoption, especially within health systems under budget constraints, involving multiple decision-makers and growing demands for evidence.
For industry leaders, the debate has evolved: it is no longer just about proving that a solution works, but about defining how to enable access in a sustainable way that aligns with the dynamics of the system in which it will be implemented.
The new market access is not linear, it is systemic
Traditional market access models, based on predictable pathways of regulatory approval, pricing, and commercialization, have shown clear limitations in the face of today’s sector complexity. Access increasingly occurs in fragmented environments, where clinical, regulatory, economic, and operational decisions overlap.
In this context, strategies that integrate from an early stage gain relevance:
- the design of clinical evidence oriented toward incorporation,
- a deep understanding of care delivery flows,
- insight into different decision-making levels, including managers, providers, and payers,
- the ability to execute in heterogeneous contexts.
Market access ceases to be a final step and becomes a transversal strategic capability, influencing development, positioning, and scale decisions.
Access challenges in the SUS: the Phelcom case
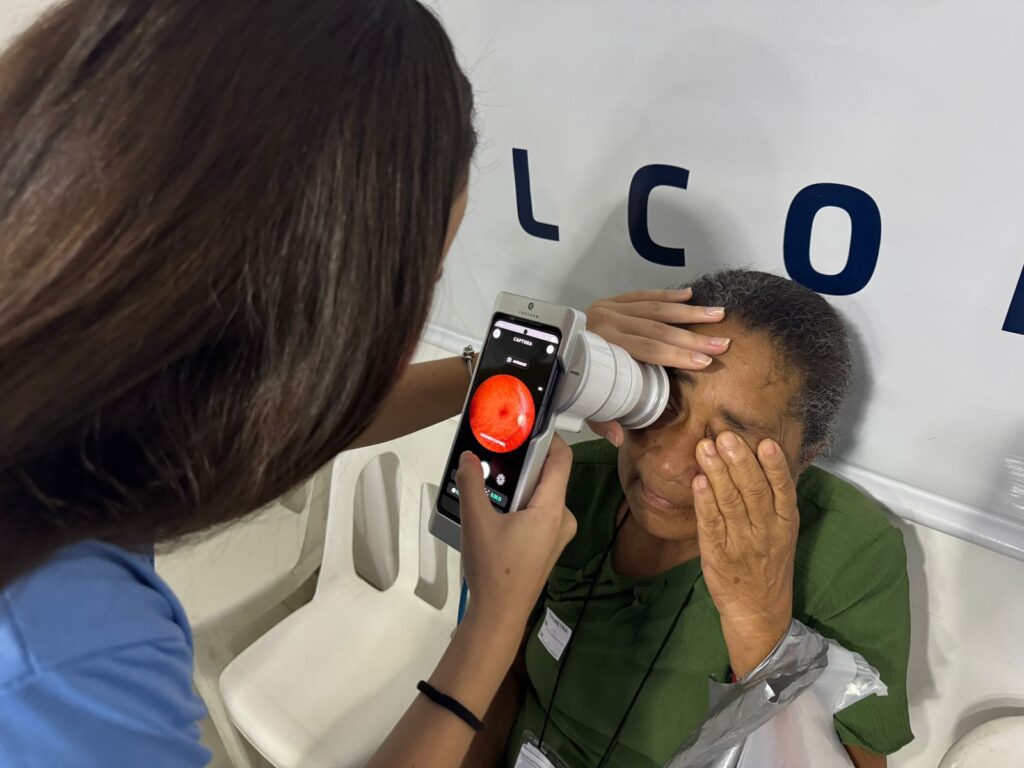
In Brazil, this complexity is intensified. The SUS presents unique challenges related to scale, operational diversity, and coordination across levels of care. At the same time, it clearly highlights a central point of market access: there is no sustainable incorporation without consistent real-world operation. A practical example of this dynamic is the trajectory of Phelcom, a startup from the Eretz.bio ecosystem specializing in the development of portable devices for visual health.
Founded by experts in physics, electronics, and computing, the startup developed the Eyer and Eyer2 retinal cameras, portable solutions integrated with telediagnosis systems that combine image quality, cloud connectivity, and support for remote clinical analysis. These characteristics enable use in decentralized settings and primary care, guiding a progressive entry into the public market through social projects, institutional partnerships, and contexts marked by care gaps, where logistics, training, and integration into care pathways are decisive factors.
Recurring participation in large-scale health campaigns, serving more than 1,000 patients per year, functioned as a real-world operational learning environment. In scenarios of high scale and care pressure, the technology had to simultaneously meet clinical, workflow, and time requirements. This type of validation, often underestimated, is central to any market access strategy in the public sector.
The maturation of this approach led Phelcom to structural public market access milestones, such as the formal integration of the Eyer and Eyer2 retinal cameras into the telediagnosis system of the Federal University of Goiás, which is responsible for the National Diagnostic Offering in virtually all Brazilian states. This move goes beyond selling technology; it involves integration into care networks, clinical governance, and the ability to operate at national scale.
Similarly, the partnership with the Mato Grosso State Health Secretariat, which resulted in the provision of 250 devices, combines equipment supply, training of primary care professionals, and integration into the routine of Primary Health Units, reinforcing a critical point: sustainable access requires a model, not just a product. This type of strategy consolidates access as a continuous process of alignment between solution, system, and public policy.
A less evident yet highly relevant strategic aspect is how Phelcom built institutional legitimacy even before achieving full commercial scale. Ongoing operation in primary care enabled the accumulation not only of clinical evidence, but also of systemic knowledge: an understanding of care flows, primary care limitations, managers’ demands, and interoperability requirements with telehealth networks.
This learning translated into product, deployment, and training decisions, anticipating classic access barriers such as professional adoption, operational sustainability, and integration into existing processes. The result is an access model that does not rely exclusively on regulatory incentives or isolated purchases, but on the solution’s ability to function within the system, at scale and over the long term.
What this trajectory shows is that market access is not built solely through formal incorporation milestones, but also through the ability to translate innovation into real-world operation within actual systems. When a solution is designed with care flows, local capabilities, and institutional logic in mind, access ceases to be episodic and becomes structural. This type of learning, often developed within the public health context, is equally applicable to organizations seeking to scale solutions in complex, regulated, and multipolar environments, including the private market and the industry as a whole.
Recurring access challenges from the companies’ perspective
Despite advances in access, many organizations still face recurring challenges, such as:
- mature solutions that do not progress toward incorporation,
- robust evidence that does not resonate with decision-makers,
- successful pilots that fail to scale,
- strategies designed without a deep understanding of the system in which they need to operate.
This happens because market access is no longer an isolated problem; it requires a systemic view, institutional coordination, and practical knowledge of real incorporation pathways, something that is rarely solved through traditional internal structures alone.
Market access as a competitive advantage
As the sector evolves, organizations that treat market access as a strategic capability, rather than merely a technical function, tend to build sustainable competitive advantage. This implies integrating science, data, regulation, operations, and strategy from the very beginning of solution development.
The central question shifts to: how can viable pathways be structured so that innovation generates real value for the system, for managers, and for patients?
More than answering this question, the challenge lies in structuring, testing, and continuously adjusting these pathways.